The world of medical science is unimaginable without medical devices. They are omnipresent. In other words, they are found everywhere – right from diagnosing and treating the ailments to helping them in enhancing the quality of life. Rise in complex surgeries, surgical procedures, growth in chronic conditions, and the ever-increasing geriatric population are the factors pushing the medical devices market towards exponentiation. This is also evident from the data released by the US FDA, which states that close to 54 novel medical devices were approved by it between 2017 and 2018.
Out of a whole of medical devices, orthopaedic trauma devices are expected to witness a major overhaul going forward. The WHO states that road accidents result in close to 1.24 Mn fatalities every single year. These fatalities could be reduced to certain extent if orthopaedic trauma devices are used from time to time. Plates and screws witness the highest demand.
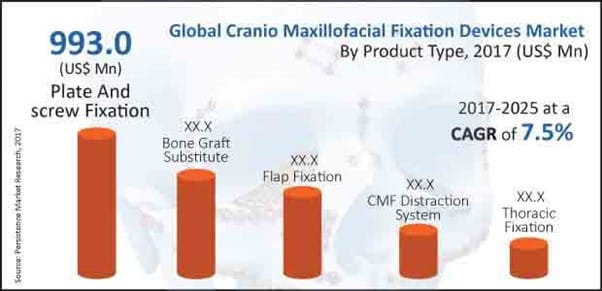
Plus, with medical practitioners shifting to minimally invasive surgeries, craniomaxillofacial devices are being asked for. They come across as multi-specialty procedure needing participation of dental surgeons, plastic surgeons, and even neurosurgeons.
Next, obesity surgery devices are there to account for the major share. This could be credited to more number of people looking upon surgical weight loss solutions as a feasible option. This factor is expected to help the obesity surgery devices market witness a double-digit CAGR between 2020 and 2030.
Microfluidic devices have found their presence in several applications like drug discovery, pharmaceuticals, POC diagnostics, biomarker analycraniosis, cell analysis, next-generation sequencing, and others. On the part of developments, Fluidigm Corporation, in August 2019, introduced automated RNA-seq workflow to be used with JUNO microfluidic system, so as to save on hands-on time as well as cost. Also, SCHOTT AG, in June 2019, announced acquiring MINIFAB Pty Ltd. (Australia-based).
Besides, not to forget – NPWT devices and dressings, better known as vacuum-assisted closure, could be single-use or traditional, which could depend upon location as well as size of wound. NPWT dressings get changed every 2 days (48 hours). They could applied on continuous basis for 2-6 weeks based on wound’s severity. Regarding developments, Smith and Nephew Plc did announce launching novel PICO 14 Single Use Negative Pressure Wound Therapy System (sNPWT) all across the US. The pump duration is enhanced in comparison with the previous variants of PICO sNPWT.
The surgery procedures mentioned above are incomplete without proper post-surgery treatment. This is where medical tapes and bandages come into picture. Plus, occurrences like “accidental burns” need to be taken care of. The WHO states that as of the year 2018, close to 1,000,000 people get moderately or severely burnt in India annually. The other countries like the UK, China, The Netherlands, Australia, and Bulgaria have reported rise in occurrence of burn wounds. All these need medical tapes and bandages with immediate effect.
Fluid warmer devices, as the name suggests, are designed to warm colloids, fluids, blood products or crystalloids, so that occurrence of hypothermia could be prevented. It needs to be noted that hypothermia is likely to cause various complications in the surgeries. They could be cardiac arrest or coma and prolonged healing time.
Persistence Market Research (PMR), with its market study on “Medical Devices Market”, has captured the key players like Fresenius Medical Care, DePuy Synthes, Medtronic, Philips Healthcare, GE Healthcare, Ethicon LLC, Siemens Healthineers, Cardinal Health, BD, Stryker, Baxter International plc, and others. PMR has gone further with mentioning that Medtronic, in June 2019, did ink an agreement pertaining to acquisition of “Epix Therapeutics” worth US$ 316 Mn. PMR also states that the same Medtronic, in June 2019, did announce acquisition of “Titan Spine”, so as to enable itself to grow the product portfolio on the count of medical devices.
Persistence Market Research (PMR), has, by geography, mentioned that North America holds the largest market share, which could be credited to it being the hub of technological advancements. Europe is second-seeded over here. The Asia-Pacific is expected to be the highest attention-seeker regarding medical devices in the years to come. This could be attributed to improvement in healthcare infrastructure.
Persistence Market Research (PMR) has categorized the medical devices market by type as cardiovascular devices, orthopaedic devices, diagnostic imaging, IVD, MIS, wound management, diabetes care, ophthalmic devices, dental, nephrology, and others. End-user-wise, it says clinics, ASCs (Ambulatory Surgical Centers), and others.